The NEW ENGLAND
JOURNAL of MEDICINE
Hilary D. Marston, M.D., M.P.H., Catharine I. Paules, M.D., and Anthony S. Fauci, M.D.
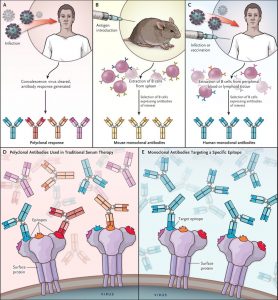
Although antibodies play pivotal roles in the immune response to infection, they have seen limited use as therapeutic agents for infectious diseases. Yet there is a long history of plasma-derived treatments for several pathogens. Emil Adolf von Behring, for example, won the Nobel Prize in Physiology or Medicine in 1901 for the application of animal-derived serum therapies, principally against diphtheria.1 Since then, plasma-based therapy has been attempted for infectious disease outbreaks ranging from the 1918 influenza pandemic to Ebola outbreaks from 1976 onward. Despite this history and the rapidly accelerating development of monoclonal antibodies (mAbs) for noncommunicable diseases such as cancer and autoimmune conditions, only a handful of antibody therapies have been licensed for infectious diseases (e.g., palivizumab for prophylaxis against respiratory syncytial virus in at-risk infants). Recent conceptual and technological advances in mAb development could have an enormous impact on the field of infectious diseases, particularly in the context of emerging infectious disease (EID) outbreaks, in which the process of vaccine development for new pathogens may be difficult and prolonged. The rapid development and strategic deployment of effective, highly specific preventive and therapeutic interventions have the potential to alter the course of an epidemic.
Research advances could facilitate a strategic shift toward the use of mAbs for EIDs. For example, unlike traditional serum therapy, which uses polyclonal antibodies, therapy using mAbs targets a single epitope, allowing for specific activity against a predetermined target (see illustration); moreover, there is the added benefit of a high degree of consistency among manufactured lots.1 Using mAbs in place of polyclonal serums has improved the safety profile of immunotherapy, reducing such concerns as the risk of serum sickness and anaphylaxis sometimes seen with animal-derived polyclonal preparations.1
In addition, effective antibodies have become easier to identify, select, optimize, and manufacture. When mAbs were originally described in the 1970s, they were derived from the vaccination of mice with antigens of interest and the harvesting of B cells from mouse spleens.1 This technique continues to have value today, but there are also more direct and improved approaches whereby relevant antibodies can be identified directly from exposed persons. For example, investigators are now able to use flow cytometry to sort memory B cells on the basis of their antigen-binding characteristics. The variable regions of the antibody heavy and light chains can be cloned and monoclonal antibodies expressed.1 From these initial procedures, candidates for mAb development can be further selected on the basis of in vitro neutralization (and other effector-function) assays that evaluate activity.1 With these and other techniques, the time required to isolate and characterize antibodies has been reduced from years to weeks.
Candidate mAbs, once developed, can be optimized with a variety of strategies, including extension of half-life through modifications to the Fc portion of the antibody molecule, an attribute that is potentially critical for prophylaxis.1 In certain settings, interactions with the Fc receptor are postulated to play a role in pathogenesis — for example, in antibody-dependent enhancement of flaviviruses. Modifications to the Fc receptor can prevent these interactions while maintaining neutralizing functions, potentially improving safety profiles.2 Finally, manufacturing techniques are improving, through use of qualified, stabilized cell lines, as well as new plant and animal systems.
With these advances, mAbs have the potential to increase our effectiveness in responding to EIDs, particularly in cases in which their use may substantially improve patient or population-level outcomes. However, given the current costs of production and relative complexity of administration (e.g., the need for parenteral administration), targeted use of mAbs will be necessary. There are three particularly compelling indications for their use: treatment of infected individuals, targeted prophylaxis to protect high-risk individuals, and targeted prophylaxis to interrupt transmission in average-risk populations.
The 2014–2016 Zaire ebolavirus outbreak in West Africa demonstrated mAbs’ promise for treatment. ZMapp, a “cocktail” of three mouse–human chimeric antibodies, had demonstrated efficacy in nonhuman primates; however, only minimal quantities were available, and it had not been evaluated in humans. In February 2015, a randomized, controlled trial was initiated in infected patients; however, as the incidence of Ebola infection dropped, trial enrollment decreased and the study failed to reach statistical significance. Still, the limited data set showed some promise of a mortality benefit.3 Since then, investigators have isolated multiple Ebola antibodies that could serve as essential tools for Ebola treatment in future outbreaks. Some of these antibody candidates may offer protection with a single mAb (as opposed to a cocktail), potentially simplifying manufacturing processes and reducing costs, and others could provide cross-species protection (which is important given the threat presented by Sudan and other Ebola virus species).4
MAbs may also play an important role in prophylaxis for persons at high risk for infection (or adverse sequelae), such as pregnant women in areas where Zika virus is circulating. Ideally, women would be protected from infection before pregnancy with a vaccine. Failing that, mAbs may have a role as passive immunization for pregnant women, offering protection for the fetus without the weeks’ delay required to induce an immune response through vaccination. Administration of a potent neutralizing antibody targeting the Zika virus envelope protein protected mice from congenital Zika syndrome before Zika virus challenge in the mother.2 This research required both the characterization of mouse pregnancy models and the isolation and production of effective antibodies, and yet results were obtained within months after the recognition of the Zika–microcephaly link in humans. Researchers hope to advance this mAb candidate into clinical trials, including studies in pregnant women. Similar individual-level prophylaxis could be envisioned for other diseases — for example, for health care workers and other high-risk populations facing outbreaks of hemorrhagic fever viruses.
Furthermore, mAbs may provide population-level prophylaxis, particularly if used to interrupt chains of transmission. For example, although vaccination is the mainstay of seasonal influenza prevention and epidemic control, it has historically had little effect on the course of evolving pandemics because of the time it takes to develop and deploy a vaccine against a novel pandemic strain. MAbs that target conserved regions of the influenza virus could bridge the time between onset of an influenza pandemic and availability of a vaccine. Several broadly neutralizing mAbs targeting the highly conserved stem portion of the hemagglutinin protein of influenza A have had promising results in preclinical studies against a range of influenza subtypes and were well tolerated in early-stage clinical trials.5 These agents have therapeutic potential and may also interrupt transmission if administered to uninfected persons who are in proximity to index cases. Transmission studies in ferrets demonstrated that administration of one such antibody, MEDI8852, to uninfected ferrets can protect them from airborne transmission of the H1N1pdm09 virus.5
Although mAbs have potential for use in responding to EIDs, pragmatic concerns must be addressed — notably cost. Targeted development and deployment of antibodies with high potency (requiring less material per dose) will help reduce the cost, as will improvements in manufacturing. Administration of mAbs, particularly those requiring cold storage and intravenous infusion, may also be challenging in some outbreak settings. In the future, the EID field may turn increasingly to high-affinity antibodies, allowing subcutaneous dosing, or novel delivery platforms such as nucleic acid and vectored constructs, minimizing the need for intravenous administration. Finally, EID pathogens probably vary in their vulnerability to mAbs, and particularly to neutralizing antibodies. Yet the knowledge gained in advancing the field of mAbs for EIDs will enable the development of other countermeasures, including vaccines, and increasingly specific diagnostics.
Despite the challenges, mAbs are positioned to play a larger role in future public health responses involving the diagnosis, prevention, and treatment of EIDs, and the lessons learned will most likely apply to infectious diseases in general. If we are to fully realize mAbs’ promise in EIDs, leaders in preparedness and response will have to assign them a high priority in research and development agendas.
From the Office of the Director of the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD.
Monoclonal Antibody Therapy.